
Press Center
Following the Wafer Foundry Model: What Piece is Still Missing in the Biotech CDMO Industry Puzzle?
Where is the next TSMC? In recent years,
Taiwan's biotech industry has been aggressively entering the Contract Development
and Manufacturing Organization (CDMO) market, sparking a wave of contract
manufacturing and being seen as the next "guardian mountain" of the
nation.
In the past, outsourcing carried somewhat
of a negative connotation. However, as TSMC has become a crucial partner in the
global semiconductor industry, it has once again proven the value of the
contract manufacturing business model. So why is production and manufacturing
also viable in the biotech industry?
In fact, Taiwan has demonstrated through
its semiconductor and IT industries how optimizing processes can accelerate
development. By the same logic, smart manufacturing might also work well in the
biotech industry.
CDMO stands for Contract Development and
Manufacturing Organization. Simply put, it refers to the outsourcing of
production services for various pharmaceuticals, including drugs, vaccines, and
medical devices. Since developing new drugs can take up to ten years, large
companies, although capable of building their own factories, often opt to
outsource production. This allows them to focus their funds on drug research
and development, control costs, and speed up time to market, making the CDMO
business model increasingly popular.
An Illustrated Guide to Understanding
CDMO
The most notable recent event in the
biotech industry is the passage of the dual laws on regenerative medicine,
which will help establish Taiwan's regenerative medicine industry.
Regenerative medicine is considered an
emerging medical treatment that mainly uses a large number of active cells or
gene-edited immune cells as drugs to treat difficult diseases. However, meeting
the demands of regenerative medicine is challenging due to the current
insufficiency of cell preparation and production capacity.
"Cell therapy is a rigid demand. If we
develop a CDMO manufacturing center, it can bring stable mass production and
quality standards to cell therapy and may help overcome industry
bottlenecks," said semiconductor giant Xu Jin-rong. It is understood that
he once suffered from nasopharyngeal cancer, which is why he is optimistic
about cell preparation CDMO. He believes that just like wafer foundries in the
semiconductor industry, CDMO will create greater value. Therefore, he has
quietly invested in biotech companies within the industry, hoping to use his
experience in semiconductor equipment to domestically produce medical equipment
and bring hope to cancer patients.
Industry insiders point out that while CDMO
is not an emerging industry, the urgent need for vaccines during the pandemic
highlighted the vulnerability of supply chains, making everyone aware of this
issue. Supply chain restructuring is happening not just in the tech industry
but also in biotech. Various countries aim to achieve supply chain autonomy,
making this the perfect time for CDMO companies to shine.
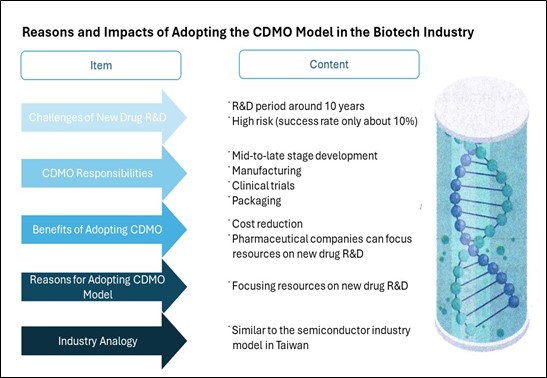
Why does the biotech industry need CDMO?
The main reason is that new drug development is time-consuming and risky, with
a success rate of only about 10%. It usually takes 10 years from clinical
trials to obtaining drug approval. To focus resources on new drug development,
pharmaceutical companies outsource the middle and later stages of development,
manufacturing, clinical trials, and packaging to reduce costs. This model is
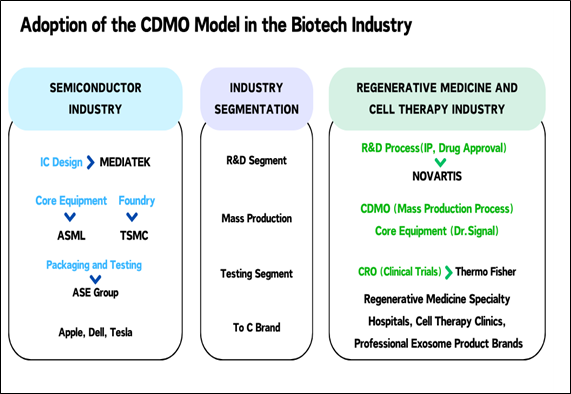
quite similar to the familiar semiconductor industry in Taiwan.
Simply put, companies like MediaTek,
Qualcomm, NVIDIA, and AMD contract TSMC for wafer foundry services, while
biotech companies or pharmaceutical firms, similar to these fabless
semiconductor companies, mainly focus on R&D. CDMO, in short, operates similarly
to TSMC's business. If a new drug is favored by a pharmaceutical company and
deemed to have market potential, the company contracts a CRO (Contract Research
Organization) to provide clinical trial services and help obtain drug approval.
In the semiconductor analogy, the role of
CRO is similar to packaging and testing companies like ASE Group and
Siliconware Precision Industries.
The difference lies in the fact that the
most direct profit for biotech pharmaceutical companies is obtaining drug
approvals from authorities like Taiwan's TFDA and various international FDAs as
soon as possible. This gives them the right to manufacture and sell drugs,
enabling large-scale production and sales. For biotech pharmaceutical
companies, securing drug approval is the most critical hurdle for new drug
commercialization. Drug approvals must be granted by health authorities in each
country, and CROs play a crucial role as key intermediaries. In contrast,
regulations and standards in the semiconductor industry are dictated by
industry leaders.
What Piece is Still Missing in the CDMO
Supply Chain?
Industry insiders in regenerative medicine
point out that once the "dual laws on regenerative medicine" are
passed, and both public and private insurance cover the treatments, the number
of patients receiving these therapies could increase by more than tenfold,
potentially making cell therapy more widespread. However, there are two
existing barriers: first, the traditional reliance on manual production, which
prevents price reduction, and second, the lack of clear division of labor
within the industry.
Typically, the more mature an industry, the
higher its division of labor. CDMO is often compared to wafer foundries, but a
closer look reveals that one reason Taiwan's semiconductor industry holds such
influence is due to its highly specialized supply chain. In contrast, the
division of labor and positioning in the CDMO of regenerative medicine and the
broader biotech industry are far less clear.
Industry insiders acknowledge that many
biotech companies in Taiwan currently handle everything from new drug
development and clinical trials to manufacturing and end-market sales. However,
one should ask, is there any semiconductor company that simultaneously manages
design, production, and packaging? Even though South Korea's Samsung
Electronics can be considered a comprehensive semiconductor company, it faces
awkward competition in AI semiconductors.
The most serious issue is that biotech
companies should not attempt to recoup their initial investments by venturing
into branding and the end market to make quick profits, akin to MediaTek
selling smartphones to push their chips directly to consumers.
Additionally, insiders note that Taiwan's
biotech research and development are impressive. However, at this stage, both
government and private resources and funding are often not concentrated. Talent
is dispersed across different small companies, often working in the same field,
resulting in unclear division of labor. For Taiwan's biotech industry to
advance, medium-sized biotech companies need to merge and integrate, or funds
should be used to consolidate several small companies into globally competitive
mid-to-large companies, thereby attracting more international investment.
Resources are limited and must be allocated correcly.
Moreover, while Taiwan is actively
developing CDMO, it is primarily responsible for mass production. The key
remains in the early-stage R&D; otherwise, the volume of orders will not
support mass production demand. Industry experts believe that instead of a
scattergun approach, resources should be concentrated, such as focusing on new
drug development, allowing both R&D and manufacturing to thrive in tandem,
achieving a supply-demand balance and stimulating healthy industry development.
Yang Yu-min, former Global Head of
Technical Operations at Roche and chairman of Yourgene Health, often referred
to as the Morris Chang of the biotech industry, also urges that Taiwan should
focus on R&D innovation-based biotech and CDMO to seize the golden
opportunity in the post-pandemic capital market.
Reasons and Impacts of the Biotech Industry Adopting the CDMO Model
CDMO Must Follow the Market
Compared to OEM and ODM commonly seen in
manufacturing, the terms more frequently used in biotech and pharmaceuticals
are Contract Manufacturing Organization (CMO) and Contract Development and
Manufacturing Organization (CDMO). The key difference is that CDMOs focus more
on product development. They work closely with clients during the development
phase, sometimes even co-developing or designing products, thus aiding more
research-focused companies in technology transfer.
Former director of ITRI's Biomedical
Technology and Device Research Laboratories, Lin Qi-wan, has publicly stated
that CDMOs have patents, understand processes, and possess expertise in their
respective fields, knowing how to add value and innovate products. With the
example of TSMC in front, the industry is optimistic about the future,
believing that "those who master the process will rule the world."
As AI enters a new wave of arms race,
Taiwan is undoubtedly the world's most important chip production base. The
recent global chip shortage has highlighted Taiwan's status and unexpectedly
boosted the performance of China Airlines and EVA Air. However, the logic of
building a cell preparation CDMO must be market-oriented. Industry insiders
state that since cell therapy requires patient-derived samples and cells are
living organisms with transport limitations, production must occur near the
patient's location.
Thus, the ideal business model is to
collaborate with large local medical institutions, leveraging their patient
absorption capacity to solve transportation issues. For CDMO companies, their
ability to export entire factories will be key to securing orders and business
development.
Furthermore, although cell therapy is an
emerging treatment, rapid development is challenging if relying solely on
manually cultured cells. Therefore, process automation and intelligence are
necessary. It is understood that combining AI, AR, and automation can increase
cell production tenfold compared to traditional laboratories, and the cost per
hundred million cells prepared is only one-tenth of traditional manual
culturing.
Earlier, NVIDIA CEO Jensen Huang remarked
that life sciences would be the next big thing and that the future lies in
bioengineering. It's not hard to understand why Huang, standing at the peak of
the AI wave, would say this. Generative AI benefits various industries,
possibly reducing the need for extensive talent, but the biotech industry, with
its high economic value, is still in its infancy in AI development. Biotech
industry insiders agree with Huang's perspective.
Historically, Taiwan has only been able to
follow international giants in the development of small and large molecule
drugs. For currently incurable diseases, regenerative medicine offers patients
a glimmer of hope and is an emerging industry. Asian countries, including Japan
and China, view the development of regenerative medicine as an opportunity to
leapfrog ahead.
Now that the dual laws on regenerative
medicine have finally passed, the industry has a new opportunity to advance.
Taiwan's advantage lies in its smart manufacturing capabilities, proven in the
semiconductor and IT industries. How to replicate this success perfectly in the
biotech industry and foster rapid development in regenerative medicine is
something Taiwan is confident about.
Chen Qi-hong, chairman of Qisda, who has
been involved in the medical industry for 20 years after transitioning from an
electronics manufacturing giant, has stated that the business strategies of
electronics and medical industries are fundamentally opposite. Developing
pharmaceuticals is difficult, but successfully bringing them to market is even
harder. Unlike electronics, where specifications are relatively uniform and
regulatory approval takes only a few months and is globally applicable, the
medical industry has high entry barriers.
Regulations such as the US FDA, Taiwan
TFDA, and EU CE each have their systems, which are not universally applicable
and are relatively strict and time-consuming. Often, even after successfully
obtaining approval, the specifications may become outdated due to the lengthy
process, potentially requiring resubmission. The long pre-market journey, with
its high costs and resources, is daunting. If downstream sales are poor and
later returns are not guaranteed, the pressure can be immense.

Source:DIGITIMES
